
Josh Mailman, President of the Northern California Carcinoid / Neuroendocrine Community, noted, “The approval of LUTATHERA ® marks an exciting day for the NET community in the United States. As a medical oncologist seeing more than 500 patients with NETs each year, I am grateful to have another tool in my arsenal.” Jonathan Strosberg, MD, Associate Professor, Section Head, Neuroendocrine Tumor Program at Moffitt Cancer Center, and NETTER-1 lead investigator, commented “There are very few effective treatment options for patients with inoperable, advanced GEP-NETs who are progressive on somatostatin analogues.
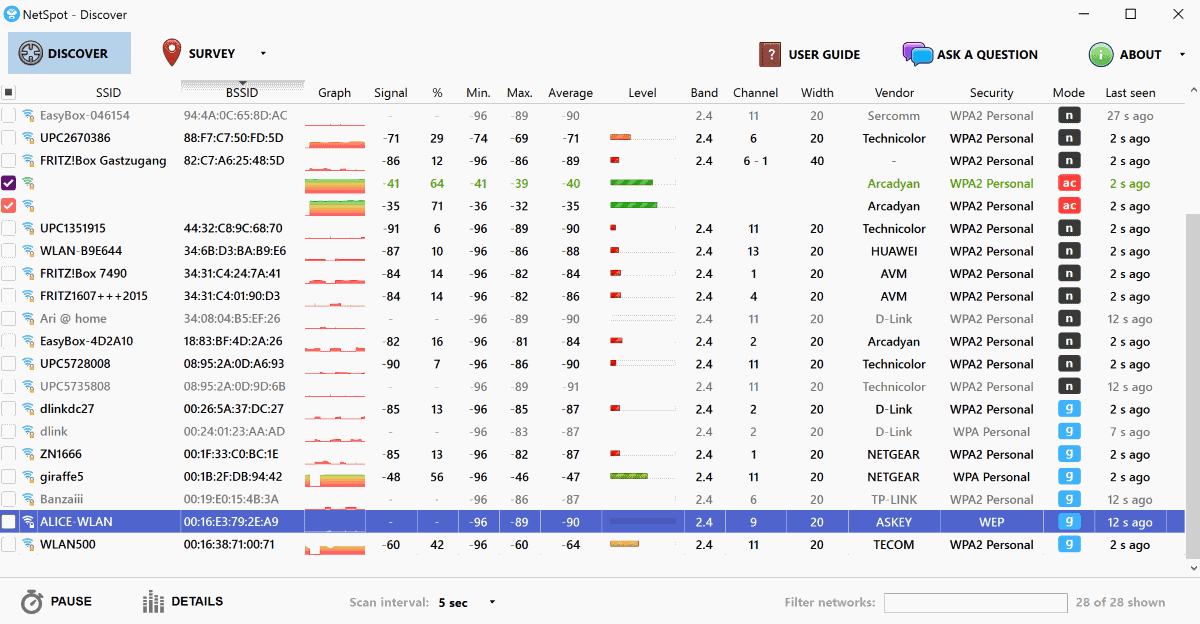
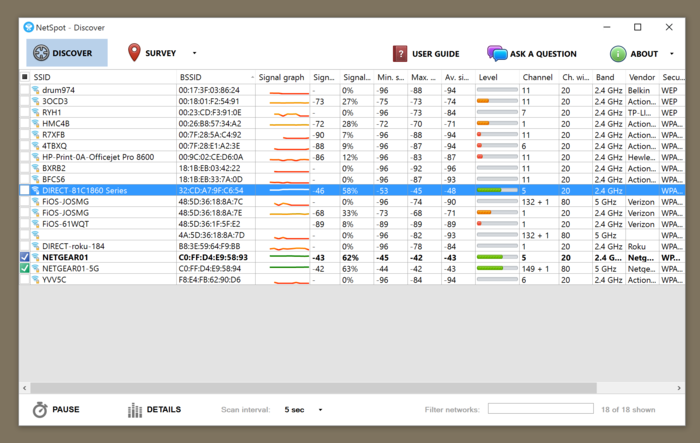
Netspot resource downloader trial#
The approval of LUTATHERA ® is based on results of a randomized pivotal Phase 3 study, NETTER-1 that compared treatment using LUTATHERA ® plus best standard of care (octreotide LAR 30mg every four weeks) to 60 mg of octreotide LAR alone (also dosed every four weeks) in patients with inoperable midgut NETs progressing under standard dose octreotide LAR treatment and overexpressing somatostatin receptors, as well as a subset of efficacy and safety data from an international, single-institution, single-arm, open-label trial conducted by Erasmus Medical Center in Rotterdam, Netherlands in more than 1,200 patients with somatostatin receptor positive tumors. The theragnostic complement to LUTATHERA ® in the US is another first-in-class drug, NETSPOT ® (gallium Ga 68 dotatate) which was approved by the FDA for localization of NETs using Positron Emission Tomography (PET) in 2016.” With this approval, AAA’s first theragnostic pairing, based on radiolabeling the same targeting molecule with either lutetium 177 or gallium 68, respectively for therapeutic or diagnostic purposes is complete. Stefano Buono, Advisor and former Chief Executive Officer of Advanced Accelerator Applications, stated, “The approval of LUTATHERA ® is the culmination of years of hard work and partnership with numerous physicians and patients. We believe nuclear medicine has the potential to offer many benefits to cancer patients and will use this approval as a foundation for the development of additional targeted cancer treatments utilizing radiolabeled ligands.”
“As the first PRRT ever approved in the US, LUTATHERA ® is introducing a major advancement in the treatment paradigm for these patients that we hope will improve many lives. “The approval of LUTATHERA ® marks an important achievement and innovation for the NET community,” said Susanne Schaffert, PhD, Chairperson and President of Advanced Accelerator Applications. Patients with well- and moderately-differentiated tumors and distant metastases have a 5-year survival probability of 35%. 1 Patient survival with advanced GEP-NETs depends on stage and histology. The estimated incidence, or rate of new cases of NETs, in the United States is approximately 6.98/100,000 per year, while the estimated prevalence for 2014, based on the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) database, was 171,321. Some patients develop symptoms arising from the excessive production of hormones by neuroendocrine tumor cells, while others remain clinically silent for years. NETs are rare tumors originating in the neuroendocrine cells of numerous organs, including the gastrointestinal tract, pancreas and lung. LUTATHERA ®, which received orphan drug designation from the FDA, is a first-in-class drug and the first available FDA-approved Peptide Receptor Radionuclide Therapy (PRRT), a form of targeted treatment comprising a targeting molecule that carries a radioactive component. (NASDAQ:AAAP) (AAA or the Company), a Novartis company and leader in nuclear medicine theragnostics, today announced that it has received US Food and Drug Administration (FDA) approval of its new drug application (NDA) for LUTATHERA ® (lutetium Lu 177 dotatate*) for the treatment of somatostatin receptor positive gastroenteropancreatic neuroendocrine tumors (GEP-NETs), including foregut, midgut, and hindgut neuroendocrine tumors in adults.

26, 2018 (GLOBE NEWSWIRE) - Advanced Accelerator Applications S.A. LUTATHERA ® marks first FDA Approval for a Peptide Receptor Radionuclide Therapy (PRRT)
Netspot resource downloader free#
First-in-class Therapy Demonstrated 79% Improvement in Progression Free Survival in NETTER-1 Phase 3 Study in Midgut NET Patients


 0 kommentar(er)
0 kommentar(er)
